Electric batteries are energy storage systems which use a reversible chemical process (i.e., a redox reaction) to store electricity. Broadly speaking, an electrical battery is a closed system that consists of four parts: (1) a positive terminal cathode, (2) a negative terminal anode, (3) a separating membrane, and (4) an electrolyte which surrounds both the anode and cathode. The negative terminal is the source of electrons that will flow through an external electric circuit to the positive terminal. When an electric battery is connected to an external electric load, the redox reaction converts high-energy reactants to lower-energy products, and the free-energy difference is delivered to the external circuit as electrical energy. Today’s modern electric batteries consists of tens or hundreds of such cells connected together in series and in parallel.
Physio-Chemical Battery Models
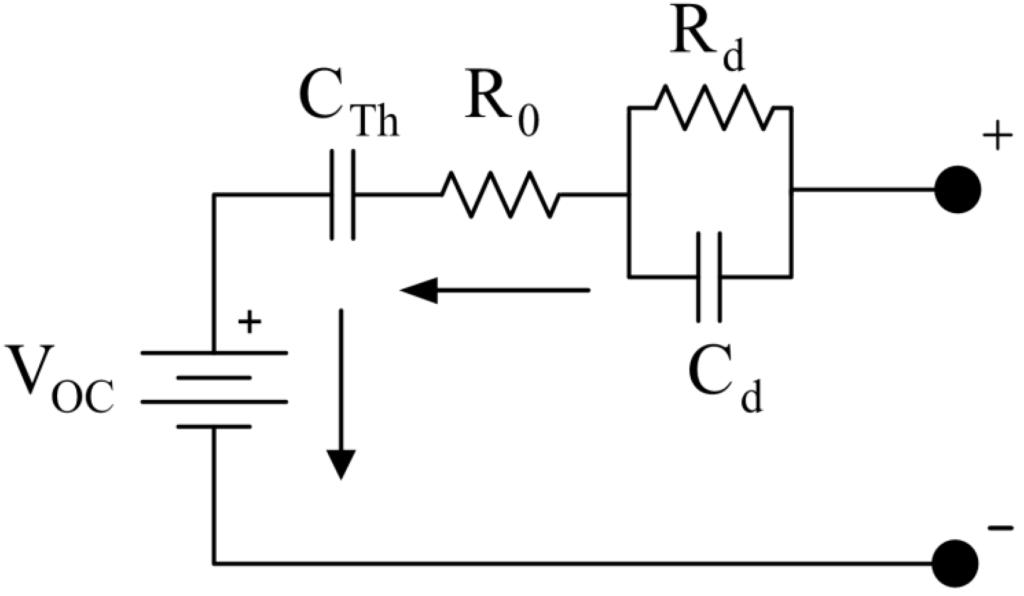
Battery models are parameterised models of the measurable variables of a battery (typically, voltage V and temperature T of (some) of the cells as well as the electrical wiring of the battery cells to form a battery pack) as (deterministic) functions of unobserved variables (e.g., state-of-charge (SoC) or state-of-health (SoH)). Modern operational battery models incorporate an (1) electrical, (2) thermal and (3) aging model in order to describe the response of a battery pack to current I applied in terms to voltage V and temperature T, particularly during their first and second life. We are investigating battery models both in terms of continuous time (i.e., the ideal model of the battery (cell)) as well as discrete time intervals (i.e., the actual measurable quantities) with the goal to infer the hidden, unobserved state of the battery (e.g., SoC or SoH) from purely operational data.